Unit 1. Metallurgy |
PART – AI. Multiple Choice Questions |
1. The metal oxide which cannot be reduced to metal by carbon is |
a. PbO | b. Al2O3 |
c. ZnO | d. FeO |
Ans: | (b) Al2O3 |
2. Electrochemical process is used to extact |
a. iron | b. Lead |
c. Sodium | d. Silver |
Ans:
| c. Sodium |
3. Considering Ellingham diagram which of the following metals can be used to reduce alumina? |
a. Fe | b. Cu |
c. Mg | d. Zn |
Ans: | c. Mg |
4. In the electrolylic refining of copper which one of the following is used as anode? |
a. pure copper | b. impure copper |
c. carbon rod | d. platinum electrode |
Ans:
| b. impure copper |
5. Zinc is obtained from ZnO by |
a. carbon reduction | b. reduction using silver |
c. electro chemical process | d. acid leaching |
| Ans: | a. carbon reduction |
6. Which of the following is used for concentrating ore in metallurgy? |
a. Leaching | b. roasting |
c. froth flotation | d. both a and c |
Ans: | d. both a and c |
7. Cupellation is a process used for the refining of |
a. silver | b. lead |
c. copper | d. iron |
Ans: | a. silver |
8. Which acts as a collector in froth floatation process? |
a. Methyl xanthate | b. ethyl xanthate |
c. pine oil | d. sodium cyanide |
Ans: | b. ethyl xanthate |
9. Zone refining process is based on ————-principle |
a. Fractional distillation | b. simple distillation |
c. liquefaction | d. fractional crystallization |
| Ans: | |
10. ———is used in making luminous paints, fluorescent lights and x-ray screens |
a. Aluminium oxide | b. copper |
c. zinc sulphide | d. zinc oxide |
Ans: | c. zinc sulphide |
PART – BII. Very Short Answer. |
1. Differentiate between minerals and ores |
- Minerals:
Minerals contain a low percentage of metal. Metal cannot be extracted easily from minerals. Clay Al2O3. SiO2. 2H2O is the mineral of aluminium. Ores: - Ores contain a large percentage of metal.
- Ores can be used for the extraction of metals on a large scale readily and economically.
- Bauxite Al2O3. 2H2O is the ore of aluminium.
|
2. Which type of ores can be concentrated by froth floatation method give two examples for such ores. |
- Sulphide ores can be concentrated by froth floatation method, e.g.,
- Copper pyrites (CuFeS2H2)
- Zinc blende (ZnS)
- Galena (PbS)
|
3. Give any two uses of zinc Applications of Zinc (Zn): - Metallic zinc is used in galvanising metals such as iron and steel structures to protect them from rusting and corrosion.
- Zinc is also used to produce die-castings in the automobile, electrical and hardware industries.
- Zinc oxide is used in the manufacture of many products such as paints, rubber, cosmetics, pharmaceuticals, plastics, inks, batteries, textiles arid electrical equipment. Zinc sulphide is used in making luminous paints, fluorescent lights and x-ray screens.
- Brass an alloy of zinc is used in water valves and communication equipment as it is highly resistant to corrosion.
|
4. Write two limitations of Elingham diagram Limitations of Ellingham diagram: - 1. Ellingham diagram is constructed based only on thermodynamic considerations. It gives information about the thermodynamic feasibility of a reaction. It does not tell anything about the rate of the reaction. Moreover, it does not give any idea about the possibility of other reactions that might be taking place.
- 2. The interpretation of ∆G is based on the assumption that the reactants are in equilibrium with the product which is not always true.
|
5. Define roasting with one example. |
- The process of heating an ore (generally, sulphide are) is strongly below its melting point in the presence of an excess of air is called roasting.
-
|
III. Short Answer | PART – C |
1. What is gravity separation process? |
- In this method, the ore having high specific gravity is separated from the gangue that has low specific gravity by simply washing with running water. Ore is crushed to a finely powdered form and treated with rapidly flowing current of water. During this process the lighter gangue particles are washed away by the running water. This method is generally applied to concentrate the native ore such as gold and oxide ores such as hematite (Fe2O3), tin stone (SnO2) etc.
|
2. Define calcination with one example
|
- It is the process in which the concentrated ore is strongly heated in the absence of air. This method can also be carried out with a limited supply of air.
|
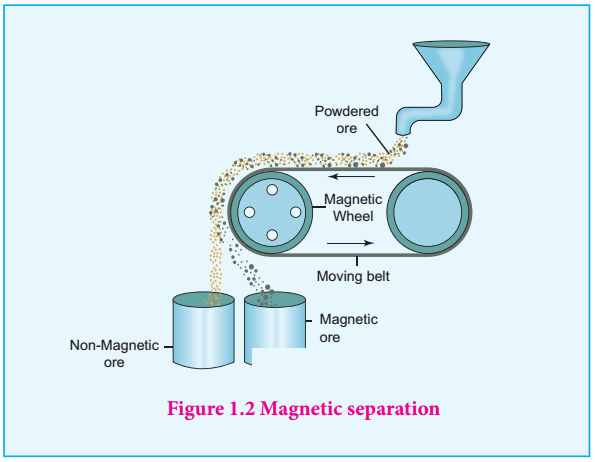
3. Write about magnetic separation process
|
Magnetic separation: - This method is applicable to ferromagnetic ores and it is based on the difference in the magnetic properties of the ore and the impurities. For example tin stone can be separated from the wolframite impurities which is magnetic. Similarly, ores such as chromite, pyrolusite having magnetic property can be removed from the non magnetic siliceous impurities.
- The crushed ore is poured on to an electromagnetic separator consisting of a belt moving over two rollers of which one is magnetic. The magnetic part of the ore is attracted towards the magnet and falls as a heap close to the magnetic region while the nonmagnetic part falls away from it as shown in the figure.
|
4. Write about electrolytic refining of silver - The crude metal is refined by electrolysis. It is carried out in an electrolytic cell containing aqueous solution of the salts of the metal of interest. The rods of impure metal are used as anode and thin strips of pure metal are used as cathode.
- The metal of interest dissolves from the anode, pass into the solution while the same amount of metal ions from the solution will be deposited at the cathode. During electrolysis, the less electropositive impurities in the anode, settle down at the bottom and are removed as anode mud. Let us understand this process by considering electrolytic refining of silver as an example.
Cathode: Pure silver Anode: Impure silver rods Electrolyte: Acidified aqueous solution of silver nitrate. When a current is passed through the electrodes the following reactions will take place Reaction at anode. 2Ag (s) → Ag+(aq) + 1 e– |
| 5. Describe a method for refining nickel |
- The impure nickel is heated in a stream of carbon monoxide at around 350K. The nickel reacts with the CO to form a highly volatile nickel tetracarbonyl. The solid impurities are left behind.
Ni (s) + 4 CO (g) → Ni(CO)4(g) - On heating the nickel tetracarbonyl around 460 K, the complex decomposes to give pure metal.
Ni(CO)4 (g) → Ni (s) + 4 CO (g) |
PART – DIV. Write in detail. |
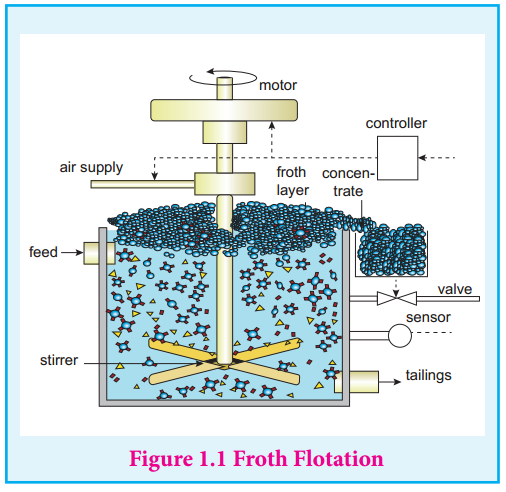
| 1. Explain froth flotation process |
Froth flotation: - This method is commonly used to concentrate sulphide ores such as galena (PbS), zinc blende (ZnS) etc. In this method, the metallic ore particles which are preferentially wetted by oil can be separated from gangue. In this method, the crushed ore is suspended in water and mixed with frothing agent such as pine oil, eucalyptus oil etc. A small quantity of sodium ethyl xanthate which acts as a collector is also added. A froth is generated by blowing air through this mixture.
- The collector molecules attach to the ore particle and make them water repellent. As a result, ore particles, wetted by the oil, rise to the surface along with the froth. The froth is skimmed off and dried to recover the concentrated ore. The gangue particles that are preferentially wetted by water settle at the bottom.
- When a sulphide ore of a metal of interest contains other metal sulphides as impurities, depressing agents such as sodium cyanide, sodium carbonate etc are used to selectively prevent other metal sulphides from coming to the froth. For example, when impurities such as ZnS is present in galena (PbS), sodium cyanide (NaCN) is added to depresses the flotation property of ZnS by forming a layer of zinc complex Na2[Zn(CN)4] on the surface of zinc sulphide.
|
| 2. Explain zone refining process |
- Zone Refining method is based on the principles of fractional crystallisation. When an impure metal is melted and allowed to solidify, the impurities will prefer to be in the molten region, i.e. impurities are more soluble in the melt than in the solid state metal.
- In this process, the impure metal is taken in the form of a rod. One end of the rod is heated using a mobile induction heater which results in melting of the metal on that portion of the rod.
- When the heater is slowly moved to the other end the pure metal crystallises while the impurities will move on to the adjacent molten zone formed due to the movement of the heater. As the heater moves further away, the molten zone containing impurities also moves along with it.
- The process is repeated several times by moving the heater in the same direction again and again to achieve the desired purity level.
- This process is carried out in an inert gas atmosphere to prevent the oxidation of metals.
- Elements such as germanium (Ge), silicon (Si) and galium (Ga) that are used as semiconductor are refined using this process.
|